The Rhizosphere is a complex system of interactions between the plants roots, soil biology and the chemical/physical properties of the soil.
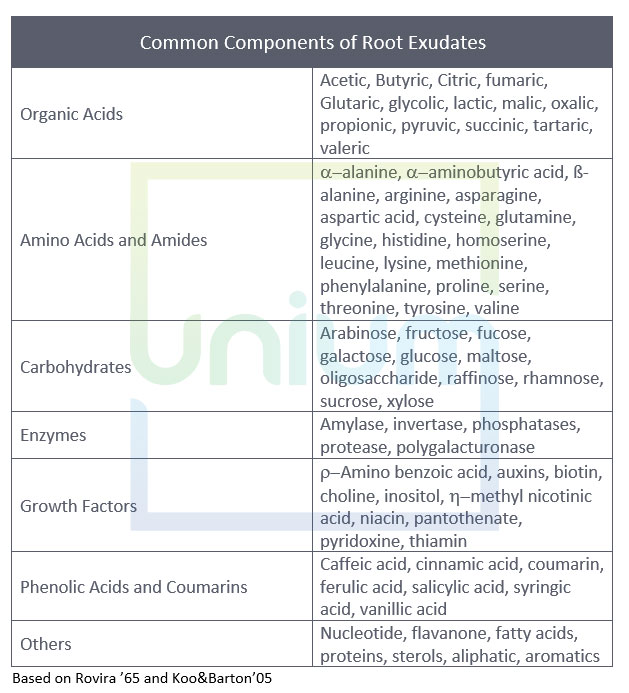
Exudates produced by roots are made up of many components that can respond to the plants needs, with organic acids, carbohydrates and amino acids being the dominant ones.
To maximise the biological interactions you need to produce high quality exudates.
Biostimulants applied at the right time and dose can maximise root efficiency through increased exudation.
 The Rhizosphere, or the zone of soil which the microbiology is influenced by the root, is a complex system of interactions – from the root to the immediate soil, to the remaining soil structure.
The Rhizosphere, or the zone of soil which the microbiology is influenced by the root, is a complex system of interactions – from the root to the immediate soil, to the remaining soil structure.
Roots excrete or exude many soluble compounds into the soil – this can be by solubilising the mucigel sheath, droplets from the root hair, shedding of the cell wall, release from the cortical cells when under attack or through root senescence.
They can have numerous functions; including providing an energy source to the rhizosphere population thus attracting the optimal rhizosphere balance and endophytic colonisation. They may also have a toxic effect on root pathogens or pests and allelopathic effects on neighbouring weeds/plants.
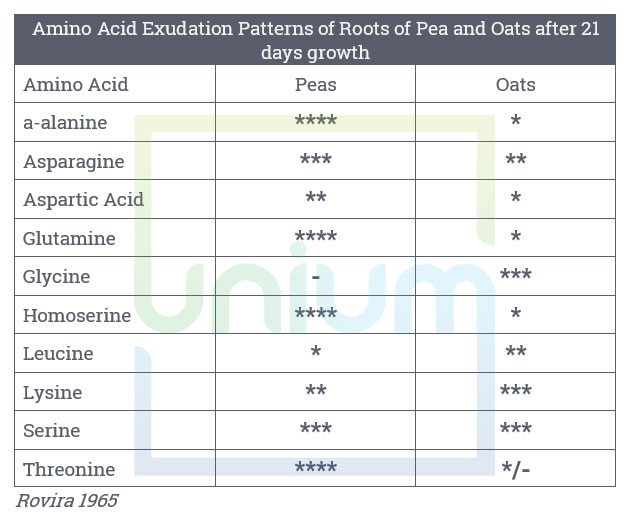
Many exudates are specific to most members of the same genus, a group of related genera or to a few species within the genera. They can be very specific in their effect on soil pathogens or symbionts. The table below highlights the difference in amino acid composition between peas and oats.
The dominate components are organic acids, sugars and amino acids. The organic acids tend to play a large role in solubilising mineral nutrients, whereas the carbohydrates are utilised as an energy source by soil micro-organisms. The non-proteinaceous amino acids enhance the mobility of plant micronutrients.
Organic acids have the strongest effect of all compounds (eg carbohydrates or amino acids) on the attraction of microbes (Lui et al ’20).
Thus, the ideal seed treatment will contain all of these components to help the plant establish in a broad range of situations.
Thus, the ideal seed treatment will contain all of these components to help the plant establish in a broad range of situations.
It’s a very complex area to study, but one thing that is clear is that composition of exudates changes under different soil conditions and during the life of the crop. For organic compounds, the root exudate content is low at the seedling stage, increasing to a maximum at flowering and then declining. Amino acids are affected by many different factors – genotype, growth stage, abiotic factors (water, temperature) and biological community of the soil. It is an area that requires a lot more research especially where amino acids are concerned as they are poorly understood. It is documented that L-Glutamate is one of the dominant amino acids, giving the largest responses for root architecture (Forde 2014) and acting in a similar way to auxin.
Organic acid exudation, such as malate and citrate, are important for solubilising and sequestering phosphorus, which when in deficiency probably has the largest effect of all nutrients on root architecture, increasing root production and root hair formation. This can be seen when using phosphite, which causes a transient phosphorus deficiency and increases root biomass, laterals and exudation making it a very valuable biostimulant in the toolbox as a seed treatment or foliar.
Exogenous application of carbohydrates, e.g. molasses, can have a similar effect to phosphites by activating a P-starvation pathway, with an important difference. They do not affect primary root growth, instead because of its strong link to sucrose and T6P, sugar accumulation often occurs in roots in response to P-deficiency (Hammon*White 2011).
More is known about the composition of root exudates than the total quantity produced, although we have seen that large quantities can be produced in our previous article “Understanding the Root Zone”.
Photo Courtesy of Ben Taylor-Davies (Regen Ben) and Rothamsted Research.
Many factors can influence this;
- Increased shoot growth increases exudation
- Nitrogen deficiency can decrease exudation
- Phosphorus deficiency can increase exudation
- Water stress and low temperatures for short periods can increase exudation
- Micro-organisms can increase exudation
- Certain herbicides increase exudation e.g. trifluralin.
Systemic herbicides released through weed roots may select microbial groups that support negative processes, such as nutrient immobilisation or support them as opportunistic pathogens. We know glyphosate increases nitrogen exudation, favouring pathogenic fungal colonisation. Some beneficials are increased with herbicide applications e.g. imazethapyr (not authorised in the UK) but it is thought the increase in beneficials occurs because the plant produces exudates, as a result of the herbicide application, to reduce the phytotoxic stress from the herbicide?
Natural varietal susceptibilities/resistances to disease and their infection can also affect the exudation profile, e.g. the infection of susceptible cotton with fusarium reduces sugar exudation, whilst in the resistant variety infection there is a minimal effect on sugar exudation. In the uninfected plants, the resistant variety exuded more amino acids when no fusarium was found (Youssef & Heitefuss ’83).
Asparagus excretes a soluble carbohydrate which is toxic to stubby root nematodes (trichodorus spp); African marigold excretes a polythienyl which is also toxic to nematodes; and beans are known to reduce wireworm populations. Flax/linseed also excretes inorganic materials, e.g. prussic acid, to protect their roots.
Allelopathic effects can generally be seen at the seedling stage and are where one species can have an effect on another species. This phenomenon can explain why small weed seedlings can sometimes effect the emerging crop more than the inter-species competition for light and nutrients.
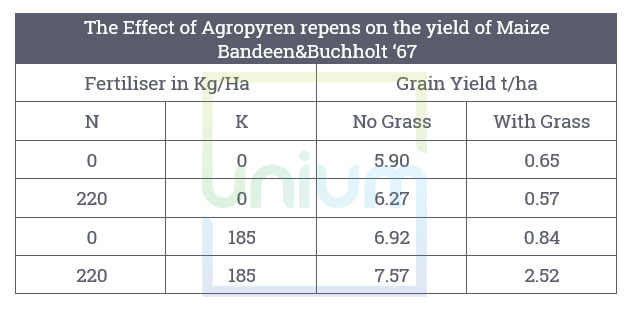
For example, couch grass releases substances that impact N and K uptake by other plants especially. maize. This allelopathic effect is not mitigated by a dressing of fertiliser at higher levels than the restriction. This trial also highlights the close relationship between N & K.
It has been shown root excretions can inhibit nitrification, the oxidation of ammonium ions to nitrite and further nitrate, in particular grasses. So not only can grassweeds have twice the root biomass of the crop competing directly, they can also exert other limitations via these exudations.
Another interesting angle is that crops can have an effect on following crops. We know of the positive effects from pulse crops leaving residual N & P behind, but certain crops will lock up soil nitrates on decomposition – a classic example is sorghum. Trials in the US showed wheat yield was 20% more after maize compared with when it followed sorghum over a 23-year period. The explanation is that the sorghum has a very high sugar content in the crown root, approx. 1000x more concentrated than the maize, and therefore locks up more nitrates as the soil microbiology feed on these sugars (there may be a negative effect on soil structure from these sugars also). Perhaps this is something to consider for game cover and cover cropping. In a rotation this can be mitigated with fertiliser or by growing a legume prior to the Sorghum.
Myers & Halsted showed oats following sorghum after lucerne provided enough N for optimal growth, but if the lucerne was replaced with soya then the yields and soil nitrate levels were reduced by a third (soya not improving soil N appreciably).
The biological associations can be very wide and varied and have multiple factors that can affect the range and populations. Within these relationships the symbiotic endophytes associations are very important.
Biologically fixed N offers more benefits than applied fertiliser, the ensuing rhizosphere acidification also promotes the increased solubilisation of phosphate. For legumes, the incorporated biomass after harvest often provides more available P from residue decomposition and solubilisation than is an equal amount of available P in the original soil form – hence pulses provide more than just N for the subsequent crop.
Often the rhizosphere can be in the order of 1 pH unit lower from biological fixation than from fertiliser application (nitrate and larger for ammonia fertilisers). This increase in acidification helps to solubilise calcium and phosphorus breaking down calcium phosphate in the soil to increase availability – we know dicots have a higher demand in general to monocots for P (but monocots do vary greatly from one species to the next).
Maize has a low Ca adsorption capacity and yields (biomass) poorly when rock phosphate is applied compared to TSP (approx. ¼), whereas millet biomass (with its high Ca adsorption rate) yields ¾ of the biomass when comparing rock phosphate application with the same level of TSP.
We know oilseed rape is capable of excreting higher levels of organic acids under low P conditions as well as increasing its root biomass (hair length), which not only solubilises phosphorus but also makes calcium available to this crop (which requires relatively high levels compared with other crops). It is relatively more efficient than maize and cow peas.
So we can see that in their natural environment, plants have a wonderful array of exudation mechanisms to cope with the ever changing environment. In modern agriculture we are able to support that in various ways – using the correct, well balanced seed treatments (Boost, Tigga, Voltek) with endophytic bacteria (TIROS) help throughout establishment and life of the crop. In season applications of phosphite, in particular the more effective and crop-safe calcium phosphite form (Calfite, aCalsa), and other key metabolites also support this vital umbilical cord from the crop to the soil via the wonderful world of soil biology.